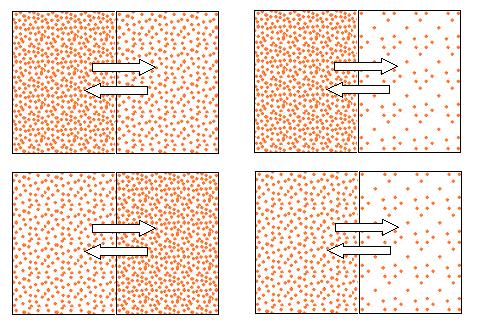
Osmosis
Because water is moving into the hyperosmolar solutions, their volume increases. This means that soon they'll be pressing against the walls of their container!
This pressure is called the osmotic pressure. So you might say that a solution is hyperosmolar to the solution next to it, or you might say that it has a higher osmotic pressure; they mean the same thing.